By replacing graphite anode with lithium metal, lithium metal battery is widely accepted as the next generation of lithium-ion battery technology. Although it has a potential to reach >500Wh/kg energy density, 60% higher than the state-of-art lithium-ion battery, lithium metal battery has not been commercialized due to high reactivity, short cycling life and vulnerability to lithium dendrites. Once its safety and cycling life issues are improved, the multi-billion US dollar market, from mAh size wearable device battery to Ah size drone battery, will gradually adopt the technology.
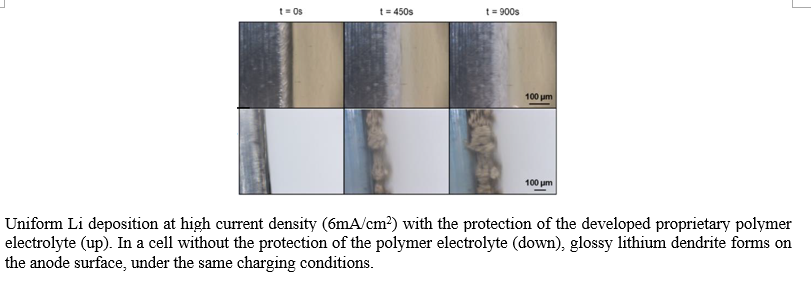
In this project, a world leading battery expert teams up with another renowned polymer chemist. They developed a high ionic conductivity polymer solid electrolyte, which has been proven in pouch cells to inhibit the growth of lithium dendrites, as well as to reduce dead lithium formation under extremely high charging rate. The complement to the polymer electrolyte is a cheap and safe carbonate-based electrolyte with conventional salt concentration. In a 1Ah prototype pouch cell with NMC 622 cathode, 50um thick lithium anode, and <3g/Ah added electrolyte, the battery retains 90% capacity after 100 cycles, at a high rate of 0.5C charge/discharge. The pouch cell could reach >350Wh/kg energy density when paired against 4mAh/cm2 high-loading NMC 811 cathode.
Material transfer and third-party testing with solid state electrolyte companies are undergoing. Collaborative full cell optimization and pilot production for consumer electronic applications are on the way with a leading lithium metal battery manufacturer.